-
8. J. Schneidewind*, Advanced Energy Materials 2022, 12, 2200342; How much technological progress is needed to make solar hydrogen cost-competitive?
Learn moreExternal linkTable of Contents Advanced Energy Materials 2022
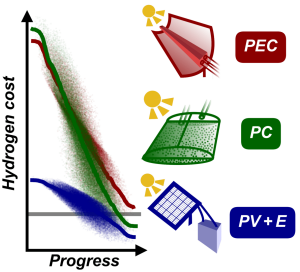
Graphic: Jacob SchneidewindCost-effective production of green hydrogen is a major challenge for global adoption of a hydrogen economy. Technologies such as photoelectrochemical (PEC) or photocatalytic (PC) water splitting and photovoltaic + electrolysis (PV+E) allow for sustainable hydrogen production from sunlight and water, but are not yet competitive with fossil fuel-derived hydrogen. Herein, open-source software for techno-economic analysis (pyH2A) along with a Monte Carlo- based methodology for modelling of technological progress are developed. Together, these tools allow for the study of required technological improvement to reach a competitive target cost. They are applied to PEC, PC, and PV+E to identify required progress for each and derive actionable research targets. For PEC, it is found that cell lifetime improvements (>2 years) and operation under high solar concentration (>50-fold) are crucial, necessitating systems with high space-time yields. In the case of PC, solar-to-hydrogen efficiency has to reach at least 6%, and lowering catalyst concentration (<0.2 g L−1) by improving absorption properties is identified as a promising path to low-cost hydrogen. PV+E requires ≈two or threefold capital cost reductions for photovoltaic and electrolyzer components. It is hoped that these insights can inform materials research efforts to improve these technologies in the most impactful ways.
-
7. A. Hamza*, D. Moock, C. Schlepphorst, J. Schneidewind, W. Baumann, F. Glorius*, Chemical Science 2022, 13, 985-995; Unveiling a key catalytic pocket for the ruthenium NHC- catalyzed asymmetric heteroarene hydrogenation.
Learn moreExternal linkThe chiral ruthenium(II)bis-SINpEt complex is a versatile and powerful catalyst for the hydrogenation of a broad range of heteroarenes. This study aims to provide understanding of the active form of this privileged catalyst as well as the reaction mechanism, and to identify the factors which control enantioselectivity. To this end we used computational methods and in situ NMR spectroscopy to study the hydrogenation of 2-methylbenzofuran promoted by this system. The high flexibility and conformational freedom of the carbene ligands in this complex lead to the formation of a chiral pocket interacting with the substrate in a “lock-and-key” fashion. The non-covalent stabilization of the substrate in this particular pocket is an exclusive feature of the major enantiomeric pathway and is preserved throughout the mechanism. Substrate coordination leading to the minor enantiomer inside this pocket is inhibited by steric repulsion. Rather, the catalyst exhibits a “flat” interaction surface with the substrate in the minor enantiomer pathway. We probe this concept by computing transition states of the rate determining step of this reaction for a series of different substrates. Our findings open up a new approach for the rational design of chiral catalysts.
-
6. J. Schneidewind*, M. A. Argüello-Cordero, H. Junge, S. Lochbrunner, M. Beller, Energy & Environmental Science 2021, 14, 4427-4436; Two-photon, visible light water splitting at a molecular ruthenium complex.
Learn moreExternal linkOverview two-photon water splitting
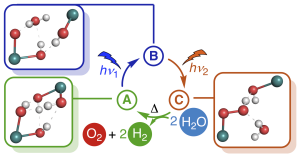
Graphic: Jacob SchneidewindWater splitting to give molecular oxygen and hydrogen or the corresponding protons and electrons is a fundamental four-electron redox process, which forms the basis of photosynthesis and is a promising approach to convert solar into chemical energy. Artificial water splitting systems have struggled with orchestrating the kinetically complex absorption of four photons as well as the difficult utilization of visible light. Based on a detailed kinetic, spectroscopic and computational study of Milstein’s ruthenium complex, we report a new mechanistic paradigm for water splitting, which requires only two photons and offers a new method to extend the range of usable wavelengths far into the visible region. We show that two-photon water splitting is enabled by absorption of the first, shorter wavelength photon, which produces an intermediate capable of absorbing the second, longer wavelength photon (up to 630 nm). The second absorption then causes O–O bond formation and liberation of O2. Theoretical modelling shows that two-photon water splitting can be used to achieve a maximum solar-to-hydrogen efficiency of 18.8%, which could be increased further to 28.6% through photochemical instead of thermal H2 release. It is therefore possible to exceed the maximum efficiency of dual absorber systems while only requiring a single catalyst. Due to the lower kinetic complexity, intrinsic utilization of a wide wavelength range and high-performance potential, we believe that this mechanism will inspire the development of a new class of water splitting systems that go beyond the reaction blueprint of photosynthesis.
-
5. J. Schneidewind*, Hrishi Olickel, Chemistry – Methods 2021, 1, 2, 89 -100; Improving Data Analysis in Chemistry and Biology Through Versatile Baseline Correction.
Learn moreExternal linkTable of Contents Chemistry Methods 2020
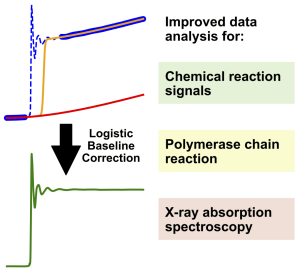
Graphic: Jacob SchneidewindAccurate data analysis is a cornerstone for the meaningful interpretation of measurements in chemistry and biology. To enable accurate analysis, it is often necessary to remove the background from a measurement via baseline correction, as is commonly done for spectroscopy or chromatography. However, no equivalent methods for baseline correction exist for an entire group of measurements, which includes chemical reaction measurements, quantitative polymerase chain reaction and X-ray absorption spectroscopy. This is because these measurements give rise to a different class of features in their signals, which prevent the application of classical baseline correction methods. In this work, a general method for baseline correction of these features is developed, which is shown to simplify and improve data analysis for these measurements. Through publicly accessible and easy to use software we expect this method to be broadly useful to improve and simplify data analysis for chemists and biologists.
-
4. D. Moock, M. P . Wiesenfeldt, M. Freitag, S. Muratsugu, S. Ikemoto, R. Knitsch, J. Schneidewind, W. Baumann, A. H. Schäfer, A. Timmer, M. Tada, M. R. Hansen, F. Glorius*, ACS Catalysis 2020, 10, 11, 6309-6317; Mechanistic Understanding of the Heterogeneous, Rhodium-Cyclic(Alkyl)(Amino)Carbene-Catalyzed (Fluoro-)Arene Hydrogenation.
Learn moreExternal linkRecently, chemoselective methods for the hydrogenation of fluorinated, silylated, and borylated arenes have been developed, providing direct access to previously unattainable, valuable products. Herein, a comprehensive study on the employed rhodium-cyclic (alkyl)(amino)carbene (CAAC) catalyst precursor is disclosed. Mechanistic experiments, kinetic studies, and surface-spectroscopic methods revealed supported rhodium(0) nanoparticles (NP) as the active catalytic species. Further studies suggest that CAAC-derived modifiers play a key role in determining the chemoselectivity of the hydrogenation of fluorinated arenes, thus offering an avenue for further tuning of the catalytic properties.
-
3. S. Kreft, R. Schoch, J. Schneidewind, J. Rabeah, E. V. Kondratenko, V. A. Kondratenko, H. Junge, M. Bauer, S. Wohlrab, M. Beller*, Chem 2019, 5, 1818–1833; Improving Selectivity and Activity of CO2 Reduction Photocatalysts with Oxygen.
Learn moreExternal linkA highly porous photocatalyst (copper on TiO2 aerogel) was synthesized and applied in aqueous CO2 reduction without using external sacrificial electron donors. For the first time, complete selectivity toward CO and improved catalyst productivity are observed in the presence of oxygen. The optimal activity is achieved in a feed containing 0.5 vol% O2 in CO2. In situ XAS, EPR, and UV-vis measurements suggest, among different Cu oxidation states, Cu(I) to be the most active species in photocatalytic CO2 reduction. Oxygen sensing of the catalyst in the presence of O2/CO2 mixtures indicated an unexpected photoadsorption of oxygen on the titania surface. We propose photooxidation of surface hydroxyl groups to be the electron source for CO2 reduction, which is supported by hydroxyl group consumption, detection of hydroxyl radicals using in situ EPR, and detection of surface peroxide species after the reaction.
-
2. T. Senthamarai, K. Murugesan, J. Schneidewind, N. V. Kalevaru, W. Baumann, H. Neumann, P. C. J. Kamer, M. Beller*, R. V. Jagadeesh*, Nature Communications 2018, 9, 1– 12; Simple ruthenium-catalyzed reductive amination enables the synthesis of a broad range of primary amines.
Learn moreExternal linkThe production of primary benzylic and aliphatic amines, which represent essential feed- stocks and key intermediates for valuable chemicals, life science molecules and materials, is of central importance. Here, we report the synthesis of this class of amines starting from carbonyl compounds and ammonia by Ru-catalyzed reductive amination using H2. Key to success for this synthesis is the use of a simple RuCl2(PPh3)3 catalyst that empowers the synthesis of >90 various linear and branched benzylic, heterocyclic, and aliphatic amines under industrially viable and scalable conditions. Applying this catalyst, −NH2 moiety has been introduced in functionalized and structurally diverse compounds, steroid derivatives and pharmaceuticals. Noteworthy, the synthetic utility of this Ru-catalyzed amination protocol has been demonstrated by upscaling the reactions up to 10 gram-scale syntheses. Further- more, in situ NMR studies were performed for the identification of active catalytic species. Based on these studies a mechanism for Ru-catalyzed reductive amination is proposed.
-
1. J. Schneidewind, R. Adam, W. Baumann, R. Jackstell, M. Beller*, Angewandte Chemie Int. Ed. 2017, 56, 1890–1893; Low-Temperature Hydrogenation of Carbon Dioxide to Methanol with a Homogeneous Cobalt catalyst.
Learn moreExternal linkHerein we describe the first homogeneous non-noble metal catalyst for the hydrogenation of CO2 to methanol. The catalyst is formed in situ from [Co(acac)3], Triphos, and HNTf2 and enables the reaction to be performed at 100 °C without a decrease in activity. Kinetic studies suggest an inner-sphere mechanism, and in situ NMR and MS experiments reveal the formation of the active catalyst through slow removal of the acetylacetonate ligands.