Topics for B.Sc./M.Sc.
Bachelor’s and master’s theses at Oschatz group
The research at Oschatz group revolves around synthesis and characterization of nanostructured and porous materials for catalysis and energy storage. Various synthetic methods for the precise tuning of particle size, morphology, and porosity meet state-of-the art characterization methods including gas-sorption, XRD, electron microscopy, and Raman spectroscopy. We strive not only to achieve high-performance materials but also to look behind the curtains and understand structure-property relationships. Interested students are always welcome to perform internships in our group, but also topics for bachelor’s and master’s theses are available and the topics can be discussed with the responsible supervisor.
-
Electrochemical Energy Storage
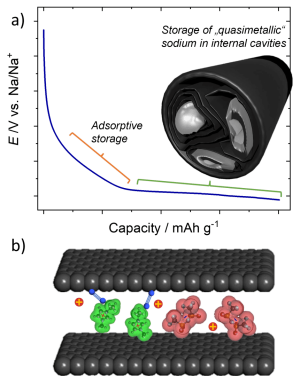
a) Charge-discharge curve of a typical negative sodium ion battery electrode material. It is desirable to extend the low-voltage plateau by incorporating internal voids in the material, where sodium can form near-metallic structures reversible. b) Ions inside of a carbon nanopore exhibiting specific double layer structures and interactions with dissolved gas molecules.
Image: Konstantin SchutjajewElectrochemical energy storage plays an important role to effectively utilize inherently volatile renewable energy sources.
Larger scale storage can be effectively achieved by battery systems and the sodium ion battery is a particularly interesting candidate due to its high energy density and abundance of involved materials. Our research on the one hand focuses on the understanding of processes occurring on the negative electrode side, trying to identify the origins of high-energy density storage, and on the other hand utilizes the gained insights to prepare materials with enhanced storage properties.On a faster time scale, supercapacitors with porous carbon electrodes can provide energy in short bursts. Here, the pores play an outstanding role, as they must have sufficient area for the adsorption of ions and at the same time provide diffusion “highways” to transport those ions in a short time. We investigate the interactions of the ions with each other and with pore walls of different width, shape, and surface chemistry to enhance energy storage in supercapacitors.
-
Electrocatalysis
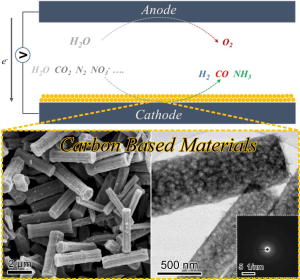
a) Overview of some electrochemical reaction catalyzed by surfaces. b) and c) metal organic framework-derived, nitrogen carbon rods used for electrochemical ammonia production.
Image: Wuyong Zhang„God made the bulk, surfaces were invented by the devil.“ – were Wolfgang Pauli‘s words on
the difficulty to model properties of solid surfaces. These structures share their borders with external media,
instead of other similar particles and here at Oschatz Group we are dedicated to take advantage of the unique
properties and possibilities arising at interfaces of porous solids to utilize them in some of today‘s most recent
challenges such as the electrochemical ammonia production or CO2 conversion and take part in materials synthesis, characterization, and application. -
Adsorption and Separation
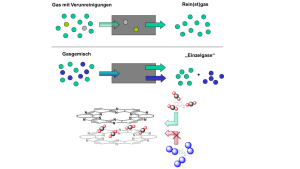
Gas purification and separation
Image: AG OschatzNanoporous materials can reach enormous specific surface areas of thousands of square meters per gram. The interaction of these surfaces (the "adsorbent") with fluid phases or compounds within fluid phases (the "adsorptive") is called "adsorption". The scientific targets of materials chemistry about such adsorption processes are versatile and range from the rapid uptake of the adsorptive over the optimization of the gravimatric or volumetric storage capacity to even the precise control over the bindung energy with the surface. All these individual processes are of upmost importance in applications like the purification of toxic exhaust gases or the removal of trace compounds from mixtures and can be controlled by the controlled synthesis of nanomaterials.
In the future, it should become possible to directly capture CO2 selectively from air as plants are able to do or to selectively remove traces of heavy metal ions or pharmaceuticals from water in the presence of other substances.
In the future, the adsorption of highly volatile gases such as hydrogen or noble gases will also become increasingly important.